Clinical Trial Management Software Buyer's Guide
Table of Contents
- What is Clinical Trial Management Software?
- How to Choose the Best Clinical Trial Management Software?
- What Are The Benefits Of Clinical Trial Management Software?
- What factors must be considered for buying the best Software?
- Questions to ask a vendor when buying best Clinical Trial Management Software
- How Much Does Clinical Trial Management Software?
- Conclusion
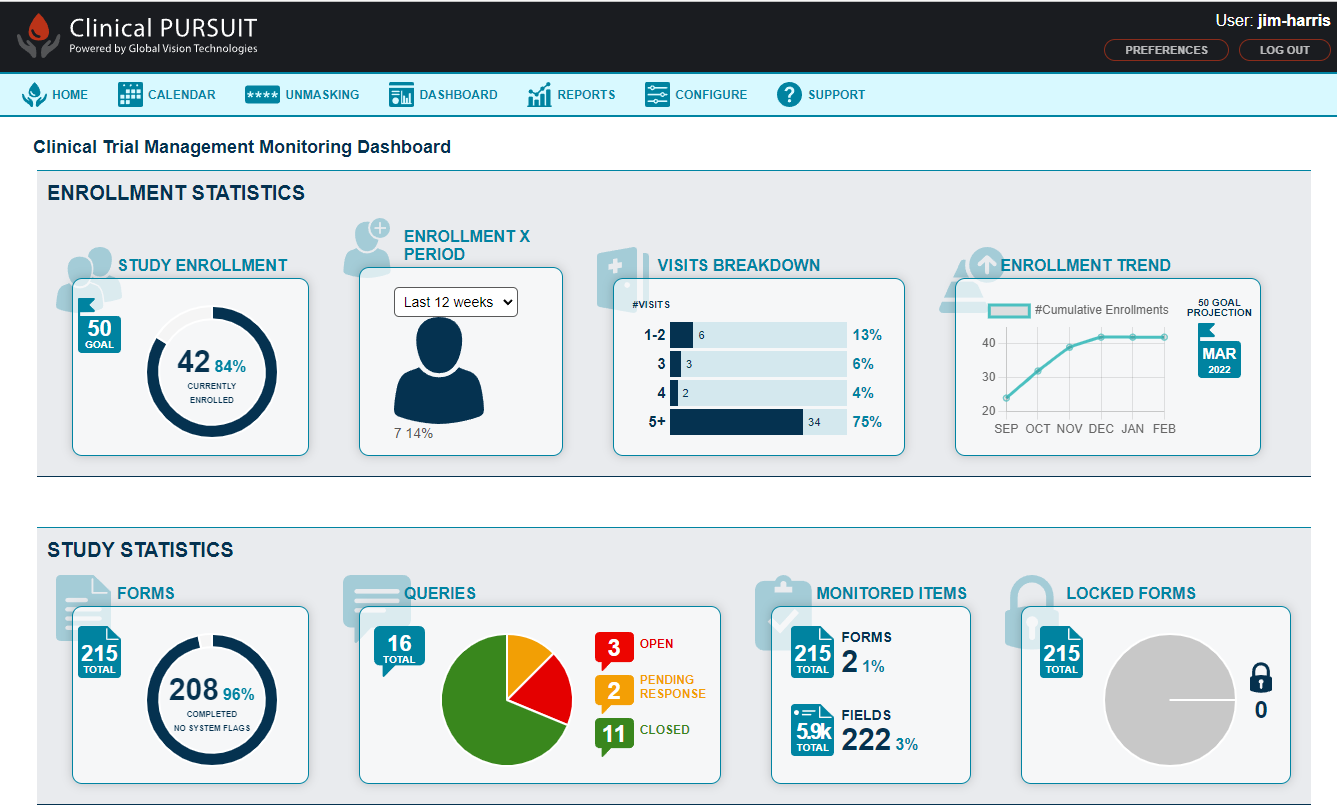
What is Clinical Trial Management Software?
Clinical trial management software is primarily used in the biotechnology and pharmaceutical industries to automate the process of clinical trials and clinical researches. It is a centralised and web-based resource that supports clinical trial processes like planning, reporting, sharing information with the concerned teams, meeting research deadlines, and taking corrective actions as and when required.
Clinic trial management software has evolved over the years leading into a sea change in the features that once were and the ones that are available now. The current features of clinic trial management software have changed due to factors like changes in the number of patients willing to undergo treatment, cost of healthcare, availability, and accessibility of healthcare services, the emergence of new markets due to globalization, the partnership between various healthcare providers, mitigation of risk, etc.
How to Choose the Best Clinical Trial Management Software?
› INTEGRATION OF STAFFING DETAILS:
The software must be customisable as per the skill set level, languages spoken, number of years of experience, location of the staff members that are ultimately going to use the software. The software must also be able to integrate the history of the team, resources allocated to each team, deadlines, milestones, achievement of results vis-à-vis the actual assigned project are important factors to consider.
› INTEGRATION WITH PAYMENT GATEWAYS:
All the different site locations have their own payment gateways. The clinical trial management software must be able to accommodate payment splitting, taxes, the ratio of payments, and other payment capabilities.
› INTEGRATION WITH CLOUD HOSTING SERVICES:
In today’s times of increase in accessibility of healthcare and the rise in population levels, the data generated during clinical trials have also increased manifolds. Existing devices and data storage facilities cannot handle the amount of data that needs to be stored and used. The clinical trial management software should be able to integrate with cloud services to deploy, scale, and use the data.
› MOBILE ADAPTABILITY:
The clinical workforce has not only increased in numbers but has also increasingly become mobile i.e. they work from remote locations or work from home. The workforce uses an assortment of devices like smartphones, tablets, notepads, laptops, etc. to access clinical data and work. The clinical trial software that you intend to invest in should have capacities to integrate with digital devices across spectrums like the ones mentioned above for it to have seamless flexibility and efficient performance.
What Are The Benefits Of Clinical Trial Management Software?
Clinical trial management software (CTMS software) centralises all the components of a clinical trial. These are some of the key benefits of CTMS software:
› EFFICIENT MANAGEMENT OF DATA:
Data is the key to any clinical trial. Data, either historical or current, helps the clinic officials in understanding the precinct of the entire clinical trial. The data generated during such a trial is humongous and extremely critical for its success. Managing this data through multiple spreadsheets and data centres is not only cumbersome but also prone to errors, which might negatively impact the findings of the trial. Clinical trial management software offers a centralised location for the entire data. Not only is this data stored centrally, but it can also be accessed easily, shared, modified, and distributed amongst the teams working on the trial. This is even more beneficial when there are multiple studies going on at the same time.
› SECURITY OF THE DATA:
The data generated during a clinical trial, especially that pertaining to the patients, is extremely sensitive and confidential. A clinical trial software ensures the security of this data by incorporating logins, user permissions, and assigning passwords to the files. This is cumbersome when one is managing multiple spreadsheets and data centres and leads to confusions and lack of communication.
› MANAGING THE FINANCES OF THE CLINICAL TRIAL:
CTMS software ensures that all the finances pertaining to a clinical trial whether the sponsorship amount or the payments and receivables are accurately managed. This is especially important when there are multiple sites involved in a particular clinical trial. The software creates a detailed and interactive schedule that breaks down the payments into logical subparts. This, in the long run, ensures better financial management and reducing any risks, which might occur making the trial more compliant to budgets and taxes.
› THE OVERALL EFFICIENCY OF THE TRIAL:
The research staff involved in a clinical trial at times gets pulled into administrative tasks that take away their precious time allocated for the research. Clinical trial software ensures that all the processes are simplified, automated, and speeded up. The staff can focus more on patient care rather than being involved in the mundane administrative tasks.
What factors must be considered for buying the best Software?
Investing in CTMS software is a huge financial decision for any clinical research organization. Installing a clinical trial management software means an overall overhaul of the processes as well. Therefore, these factors should be considered critically before investing in this software:
› FULFILMENT OF OBJECTIVES:
For this, you need to define what are your actual objectives of investing in a CTMS software. Unless you are clear about your objectives you cannot invest in software optimised to fulfil those. You might be looking at objectives like simplifying the process, to reduce costs, securing your data etc. Lay these down before purchasing the software.
› EASY INTEGRATION:
You need to assess if the software is easy to integrate into your existing systems, teams, and procedures. If your end user i.e. the teams are not able to use it, it is going to be a wasteful expenditure. Involve your teams in the purchase decision and let them experience a demo of the software. It will lead to fewer problems at a later stage.
› SERVICE FROM THE SOFTWARE PROVIDER:
Once you purchase and install the software does the real challenge start. For a smooth transition and adoption of the software, it is important to have after-sale support from the provider of the software along with training material and sessions to make your teams comfortable with the software.
Questions to ask a vendor when buying best Clinical Trial Management Software
1. Will your clinical trial management software help manage my compliance needs?
2. Is the system capable of managing site performance?
3. Will the solution help define protocols along with their management?
4. Does the system offer recruitment and monitoring of patients?
5. In what ways will the system simplify patient management?
6. Does the software provide scheduling functionality, for instance, scheduling of patient visits?
7. Does the product facilitate the import and export of data?
8. Is there a way to manage all the documents from a single place?
9. Does the software extend support to Electronic Trial Master File (eTMF)?
10. Does it allow tracking of status in real-time?
11. What about EMR/EHR?
12. Will the software help manage workflows as well as the allocation of work?
13. What does your product offer when it comes to payment systems and contracts?
14. Does it offer on-site audits and audit trails?
15. Will the system send automated reminders and updates?
16. Does the software include any functionality to verify the source of data?
17. Will it aid in the planning and tracking of clinical training?
18. Can I plan tasks and project milestones through this system?
19. Is it easy enough to design trials?
20. Does the software provide EDC integration and third-party integrations?
21. What does your product have to offer in terms of risk identification, analysis, and monitoring?
22. What are the software’s analytics and report generation capabilities?
23. Can it help forecast budgets?
24. Is your software scalable?
25. Is it possible to customize your solution?
26. What measures do you take to ensure security?
27. Will you provide on-site, ongoing support? Is it included in the pricing plan?
28. How can I reach out for support?
29. How often do you launch new releases and upgrades?
How Much Does Clinical Trial Management Software?
The cost of clinical trial software depends on many factors like its availability (trial based or user based), implementation costs, is training included in the package, support and maintenance levels, licensing, available updates etc. Broadly the cost ranges between &1,500 to $3,000 per user depending on the package and features.
Conclusion
The clinical trial software has become essential for the success of any clinical research. We suggest you understand the kind of resources available in the market, compare their features, and make an informed investment decision for attaining maximum returns on your investment and enhancing patient care services.